Using Unapproved Medicines in New Zealand
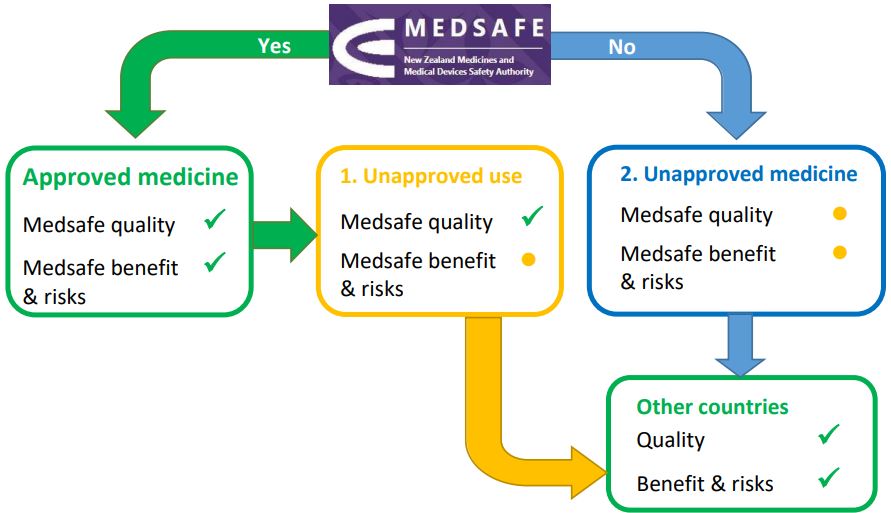
In New Zealand, a government agency called Medsafe approves medicines for people to use. They check that the quality, benefit, and risks of the medicine are what the drug company says they are. Doctors can prescribe a medicine for you outside of Medsafe’s approval in two ways:
1. Using a medicine in a way that hasn’t been approved
The medicine is used in a different way than the drug company asked Medsafe’s approval for, such as using a different dose or for a different health condition or using it for children. Your doctor or pharmacist may call this off-label use.
2. Using a medicine the drug company hasn’t asked Medsafe to approve
A medicine may have been used a lot in other countries, but the drug company hasn’t applied to Medsafe to approve it for use in New Zealand. Your doctor or pharmacist may call this a Section 29 medicine. This is because Section 29 of the law called the Medicines Act lets them give you this medicine without it being approved by Medsafe. The medicine still has the same benefits and risks, but it hasn’t been through the Medsafe process to check its quality.
Has my medicine been approved by Medsafe?
It’s a good idea to talk with your doctor or pharmacist about the benefits and risks of any medicine you are prescribed.